What is Light and where does it come from?

Photo by Nadine Shaabana on Unsplash
Introduction – What is Light?
We non-scientists take for granted that light is just light, and hardly think about where it comes from or how it’s produced. So if your work involves light, lighting or even indoor farming, we thought you might like to know.
This article pulls back the curtains on “What is Light?”.

Fig 1 Photo by Umberto on Unsplash
Light is a release of energy.
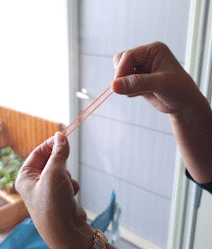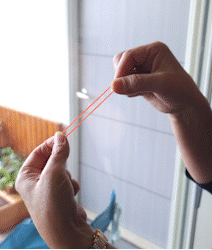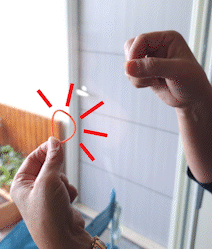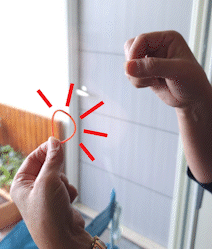
In short, photons of light that are being emitted from a light source (e.g. incandescent bulb) is simply a release of energy. To explain this lets take an analogy of a snapping rubber band.
Let’s say you apply physical energy to pull the rubber band apart. This imparts the energy into the rubber band material as potential energy. When you release one end, the rubber band contracts quickly and you hear a SNAP! The snap is the potential energy released in the form of sound.
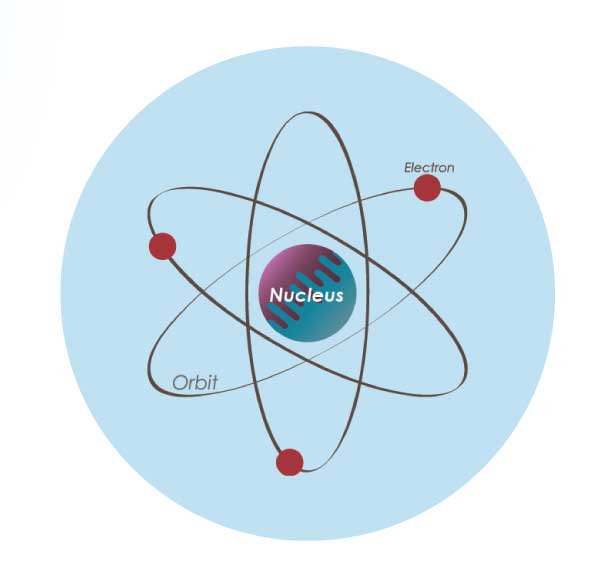
Now let’s talk about light. The filament in an incandescent bulb is made up of a material called Tungsten. Tungsten is made up of molecules, which are a bunch of atoms connected together. The atom has a center called a nucleus, and there are electrons that orbit around it.
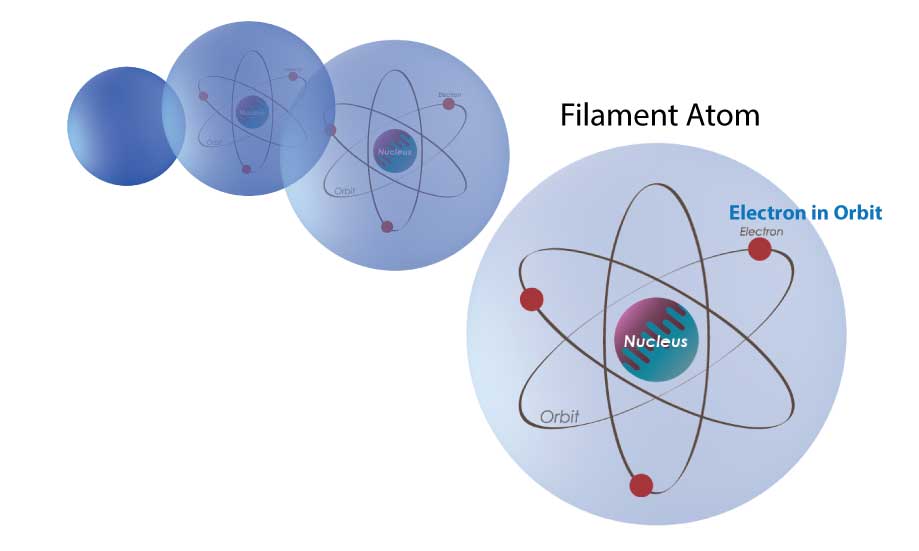
Fig 3a Filament in a Light Bulb
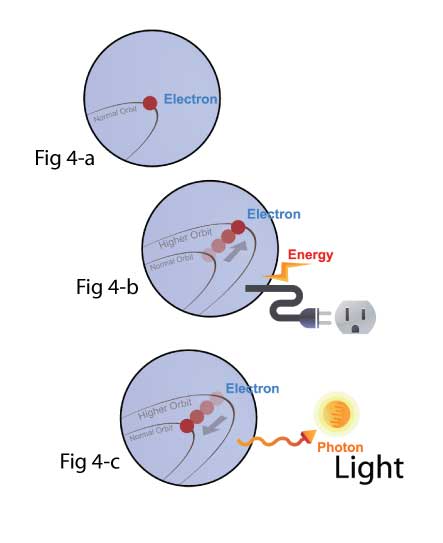
Let’s start with the electron in it’s natural low orbit (Fig 4-a).
When you apply energy or electricity to the bulb, the electron in the atom of the filament will be excited to a higher orbit and will have obtained potential energy (Fig 4-b).
When the electron snaps back to its lower state or natural state (Fig 4-c), it will release the energy – But instead of a sound, it releases a photon of light.
Ergo, light is simply a release of energy – And though other types of lights (LEDs, fluorescent, etc.) differ in many ways, they still have the same end result of emitting photons of light by allowing electrons to fall from higher orbitals or states to lower ones.
Electrons don’t stay in a higher orbit, even with constant electricity.
Why don’t electrons continue to stay in a higher orbit – after all, constant electricity is being continuously applied.
Electrons in high orbit are like someone on a high wire (tightrope). They are always in a very precarious, unstable state, and it’s easy for them to fall out of high orbit. But why are electrons unstable at this state?
Physics and Quantum Mechanics
In short, subatomic particles like electrons are weird. They live in the world of the very small, the world of Quantum Mechanics. This means that things don’t behave the same as our own worldly existence of touch, hear, see, and smell.
The very existence of an electron at any one location in time is uncertain, making its position on the high wire precarious. So, they easily fall and emit a photon of light.
And because of the constant electricity that is applied, the next electron is elevated to the higher state, and it soon falls off – and it is this continuous rise and fall of billions of electrons that gives us this stream of light from a light bulb.
Classical and Quantum view of electrons (deep dive).
Some will argue that my classical description of the atom, nucleus and orbiting electrons is inaccurate. I will concur, but this is how it’s still being taught in classrooms to help students “ease” into the complexities of physics and chemistry.
In the Quantum world, the electron exists as an electron cloud. This infers that the electron is forever popping in and out of existence around the nucleus but is never in one place unless observed.
Don’t worry if you don’t get it – even our brightest scientists have yet to unravel Quantum Mechanics completely. But that’s why electrons remain weird, and that’s why their position in time and space will always be precarious.
Light is both particle and wave
It’s easy to perceive light as particles because of its behavior: It bounces off walls and mirrors, much like a tennis ball would. Though we can’t see the particles of light, we deduce they are because of their behavior.
Light, at the same time, is also a wave, not that we can see light as watery-like waves, but because of the behavior. There was a famous experiment called the double-slit experiment where light was sent through two slits and the outcome was surprisingly similar to how water waves goes through those same two slits. Because light behaved as both particle and wave, it was deemed light was both.
Using a spectrometer to distinguish particle and wave
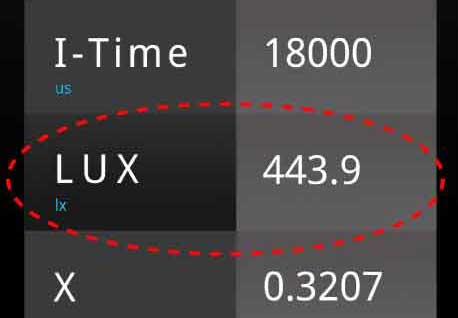
A light meter can measure and see both particle and wave aspects of light. Light is made of particles called photons. A spectrometer or light meter can measure the number of photons coming from light by measuring LUX or PPFD (grow lights). The higher the LUX, the more photons are hitting a square meter.
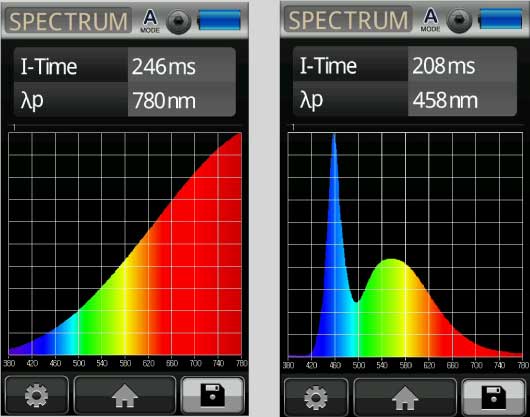
A spectrometer is a sophisticated light meter that can also measure waves, and these waves have a frequency associated with a specific color. Bluer colors have a shorter frequencies and Red colors have longer frequencies.
Each photon has an associated wavelength frequency, which determines its color, and a spectrum taken by the spectrometer can show the relative number of photons for every visible color in a light.
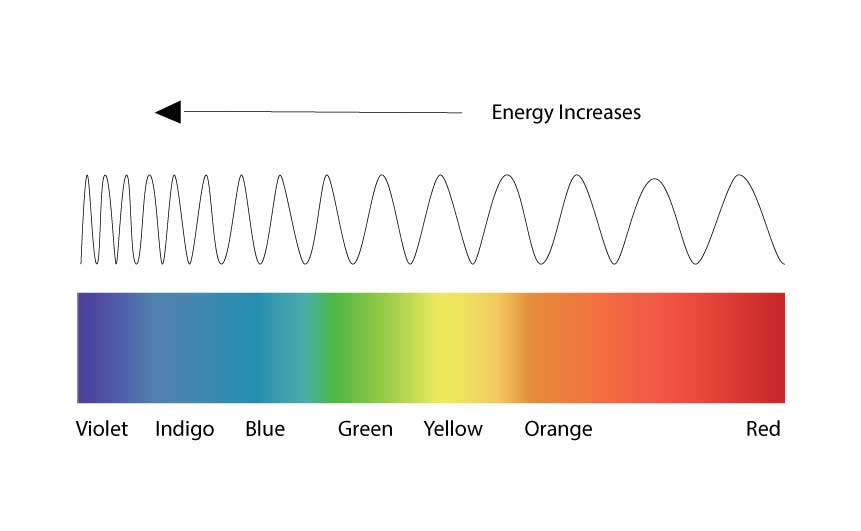
Fig 11 Bluer colors have shorter frequencies, Red has longer
Conclusion – Pulling back the curtains on What is Light.
Light is a release of energy, just like a snapping rubber band. You have to understand orbiting electrons that get pushed to a higher state. Because of the uncertainties of their existence, electrons easily fall back to their natural state, releasing energy as photons. This continuous rise and fall of billions of electrons produces a stream of light that we don’t need to take for granted anymore.
Anyone can be a scientist!

Fig. 12 All lighting uses falling electrons to produce photons of light.

Fig 13 – MK350S Premium Full Featured Spectrometer
MK350S Premium is UPRtek’s full featured Spectrometer.
Light-critical projects are challenged with awkward instrumentation that lack accuracy, flexibility, convenience, data storage, and connectivity to other devices.
MK350S Premium Handheld Spectrometer is an all-purpose, lab-grade device used by Researchers, Teachers, Lighting Designers, LED manufacturers, Light standards organizations, featuring a full lineup of features and lighting metrics to tackle any lighting challenge under the sun.
Hot Product
2 Comments
Submit a Comment
Handbook Series

The Flicker Handbook
Everything thing you need to know about Flicker, an insidious, potentially serious lighting artifact impacting visual safety for public places like hospitals, offices, libraries, and more...
About UPRtek

United Power Research and Technology
UPRtek (est. 2010) is a manufacturer of portable, high-precision light measurement instruments; Handheld Spectrometers, PAR meters, Spectroradiometers, Light Calibration Solutions.
UPRtek HQ, R&D and manufacturing are all based out of Taiwan, with Worldwide representation through our certified Global Resellers.
Latest Articles
Category












This is an interesting topic!! How does a spectrometer differentiate between the particle and wave aspects of light, and how can the measurements of LUX or PPFD alongside spectral data provide insights into both the quantity and quality of light in applications such as horticulture?
Hi Jessica – In a spectrometer light is first diffracted or separated into different colors of light, very similar to a prism or rainbow – that’s how the spectrometer differentiates the wavelengths of colors. Those individual colors of light are sent to separate sensors which record the electrical intensity or energy – these energies are converted to LUX or PPFD using algorithms, very similar to converting lbs to kg, or feet to meters – and that’s the quantity or particle aspect.
PPFD or PFD are the metrics used for horticulture – it is basically the intensity of light or particles (photons) of light hitting a surface area – this is the ” quantity of light” measurement. Suppose there is not enough quantity of photons shining on a plant in a given time period. In that case, photosynthesis will not produce enough sugars for a plant to perform metabolic duties like growth or flowering.
The Quality of a grow light refers to whether it provides light in the colors that the plant needs. In general, green plants react to blue light, red light, far red light and ultraviolet light in certain quantities, at various intervals. If a grow light provides the right colors of light in the right quantities, photosynthesis happens, flowering and fruiting happens, and plants are healthy and happy.
Spectrometers for Horticulture made with “Spectral” capabilities are called Spectral PAR meters, contrasted to traditional Quantum PAR Meters which cannot distinguish colors – they just provide PAR data.